Keywords: carbon dioxide generalities CO2 gas turbine engines captation compression injection loop circuit solvent energy recovery fossil fuel production hydrocarbon refining combustion
We are interested, more particularly, in this chapter in carbon dioxide but also in sulphur and nitrogen oxides which are often associated with the first gas. These gases are sometimes designated, in the presence of water, by acid or sour gases because of their aggressiveness on materials. They often result from the combustion of fossil fuels, mainly carbon dioxide and nitrogen oxides (also called NOx). Carbon dioxide is also produced during the extraction of fossil fuels sometimes in association with hydrogen sulphide.
These gases are very dangerous to the health of living beings but also to the environment. It is therefore necessary either to limit their production or to capture them during their extraction or during their production, in particular, following fossil fuel combustion.
Fossil fuel production and hydrocarbon refining
During the production of fossil fuels, carbon dioxide and hydrogen sulphide are separated from hydrocarbons by physico – chemical processes. In the case of hydrogen sulphide, this gas is sometimes transformed for the production of sulphur. Carbon dioxide is sometimes utilised by the food industry or re-injected into the soil either to increase the production of oils (hydrocarbons in liquid form) by raising the reservoir pressure or for long-term storage. They may also be disposed in depleted reservoirs with an aqueous phase permitting the dissolution of the sour gases into the water.
http://yvcharron.com/index.php/acid-gases-and-water-disposal/
During the refining of hydrocarbons, the sulphur fraction is greatly reduced so as to limit the emissions of sulphur oxides into the atmosphere during the combustion of fuels, mainly heavy fuels.
Hydrocarbon combustion
The combustion of hydrocarbons generates carbon dioxide, an essential molecule in the process of energy or heat production. In fossil fuel engines, the higher the production of carbon dioxide (therefore, the lower the production of carbon monoxide), the higher the efficiency in energy production. The combustion of hydrocarbons also generates nitrogen oxides, the rate of which is mainly dependent on the combustion temperature and the transit time in the combustion chambers. There are several ways to limit the NOx level. This point is not described in this document.
The rate of production of carbon dioxide per fuel mass unit or energy unit is dependent on the constitution of the carbon chain, in the sense, that the richer in atoms of hydrogen, the molecule grouping carbon and hydrogen atoms, is the more energetic the combustion is. Thus, the combustion of methane (CH4) is more energetic than that of ethane (C2H6) which is itself more energetic, successively, than propane (C3H8), butane (C4H10), pentane, hexane, etc. For the same reason, a gasoline is more energetic than a fuel or a diesel, the chains of C8 containing relatively more atoms of hydrogen than the chains of C13. The energy efficiency of a fuel combustion should not be confused here with the combustion efficiency of an engine. Thus, a diesel engine is more efficient than a gasoline engine because of the combustion temperature and not of its fuel quality.
Combustion engines
Combustion engines, whatever they are combustion turbines, diesel or gasoline engines, present a relatively low thermal efficiency, generally included between 30 and 45 percent. This means that a lot of heat, therefore energy, is rejected into the atmosphere. This energy waste is limited in large power plants by the use of a cogeneration system or an equivalent mean. There are no equivalent systems for vehicle engines for practical reasons despite the large amount of energy which could be recovered in all systems with low thermal efficiency, particularly, the ones presenting a high exhaust gas temperature (often greater than 500 °C).
http://yvcharron.com/index.php/fuel-engine-vehicles/
The high exhaust gas temperature and the large amount of heat (energy) rejected into the atmosphere could be used to implement a vessel with a temporary high pressure and low temperature permitting the dissolution of sour gases (carbon, nitrogen and sulphur oxides) in a physical solvent. Further to the sour gas captation, the purified gas could be expanded to compensate the temporary pressurisation of the polluted gas. This schematic is described in the following section:
http://yvcharron.com/index.php/fossil-fuel-power-plants/
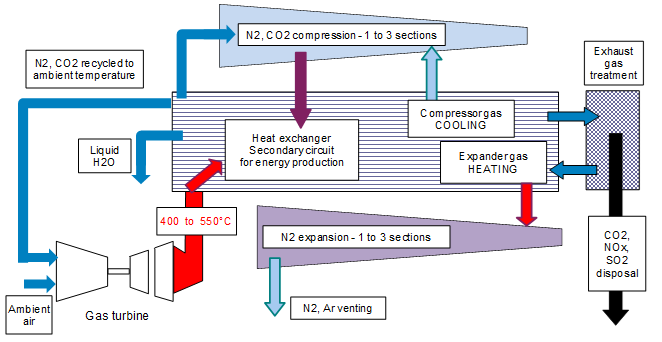
This solution for capting sour gas could be implemented at first in large power plants (totalizing several hundreds of MW where the gas pressurisation – expansion could be very efficient) including also boilers for the production of water steam activating a steam turbine. It could later be implemented in mobile engines of a large size (boats, trains, trucks and buses). With the help of miniaturization the design could be also employed in vehicles of a lower size.
Combining energy recovery and sour gas captation without a major impact on the energy recovery could be an extremely attractive solution for the environment.